A “tremendously exciting” US coronavirus vaccine that may offer very high levels of protection against Covid-19 could arrive in the UK as early as next spring, the Government has said.
British scientists have hailed the news that US firm Moderna’s jab may be 94.5% effective against the illness.
Interim data suggests the jab is highly effective in preventing people getting ill and may work across all age groups, including the elderly.
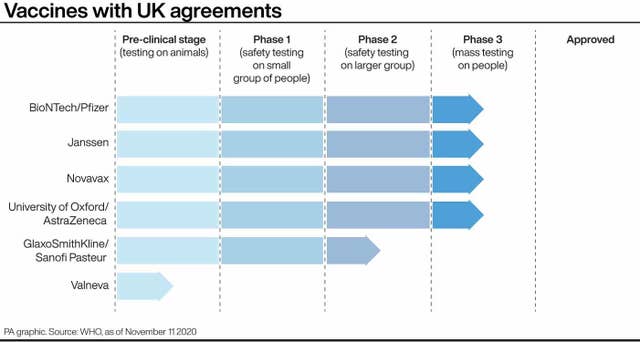
The UK has already secured 40 million doses of a vaccine from Pfizer/BioNTech, which uses the same technology as Moderna and should be in the UK before Christmas.
But it did not place orders with Moderna at the same time.
A Government spokesman said: “The news from Moderna appears to be good and represents another significant step towards finding an effective Covid-19 vaccine.
“As part of the ongoing work of the Vaccines Taskforce, the Government is in advanced discussions with Moderna to ensure UK access to their vaccine as part of the wider UK portfolio.
“Moderna are currently scaling up their European supply chain which means these doses would become available in spring 2021 in the UK at the earliest.”
Scientists said the news bodes well for other Covid-19 vaccines, with the one for Oxford University and UK pharmaceutical giant AstraZeneca due to report in the coming days or weeks.
Moderna intends to submit an application for an emergency use authorisation with the US Food and Drug Administration shortly and will submit further data on the vaccine’s effectiveness and safety.
The firm’s final-stage clinical trial is ongoing and includes more than 30,000 people in the US.
The early-stage, interim analysis included 95 participants with confirmed cases of Covid-19, of which 90 had received the placebo and five the active vaccine.
The 95 cases included 15 older adults – aged 65 and over – as well as 20 people who were not white, including 12 from Hispanic or Latino/a backgrounds, four African-Americans, three Asian-Americans and one who was multiracial.
Severe cases of coronavirus were also examined, including 11 in the first interim analysis.
All 11 cases occurred in the placebo group and none in the group which had received the vaccine, known currently as mRNA-1273.
We just announced that mRNA-1273, our COVID-19 vaccine candidate, has met its primary efficacy endpoint in the first interim analysis of the Phase 3 COVE study.Read more: https://t.co/vYWEy8CKCv pic.twitter.com/YuLubU1tlx
— Moderna (@moderna_tx) November 16, 2020
Moderna said its available data does not indicate any significant safety concerns.
However, the 94.5% efficacy from this analysis could drop as further results from the clinical trial are announced.
Dr Stephen Hoge, president of Moderna, said he “grinned ear to ear” when learning about the potential efficacy of the vaccine.
He told BBC News: “When we got the news from the data and safety monitoring board. I’ll admit I broke character and grinned ear to ear for a minute.
“Because I didn’t expect, I don’t think any of us really hoped that the vaccine would be 94% effective at preventing Covid-19 disease, that was really a stunning realisation.”
He said combined with data suggesting it can stop severe Covid-19, it means “that the vaccine really is a terrific tool for stopping the pandemic and hopefully stopping the worst of the disease that people are facing”.
He added: “When you combine it with the news of last week of Pfizer’s vaccine, you’ve got now two vaccines that are over 90% effective.

“It really means I think we have the tools necessary to finally beat this virus back and I think that’s probably the best news of the day for all of us, is that there really are now solutions in our hands and we need to deliver them to the people who can use them.”
Dr Hoge told BBC Radio 4’s World At One programme the firm was “looking forward to being able to supply substantial quantities of the vaccine to the UK Government”.
Pressed on whether the rollout could be done quickly, he replied: “It depends a little on concluding those negotiations. I don’t want to get too far ahead of ourselves.
“But we do have the ability to supply in the early part of next year and certainly we hope substantial quantities by the spring.”
Number 10 said the UK had worked on agreements with vaccine developers who offer “different types of vaccines, can provide early supply to the UK and have advanced manufacturing supply chains in place.”
At the end of October, Moderna announced that the UK’s Medicines and Healthcare products Regulatory Agency (MHRA), which approves jabs, had started the rolling review process of its vaccine.
Dr Charlie Weller, head of Vaccines at Wellcome, said: “Hopes of ending this pandemic rest on having effective vaccines, treatments and tests.
“It is incredibly promising that the vaccines we urgently need are now on the horizon.
“To have multiple vaccine candidates with interim results that surpass our expectations is phenomenal, and testament to the incredible global research effort this year.”
Peter Openshaw, professor of experimental medicine at Imperial College London, said: “This news from Moderna is tremendously exciting and considerably boosts optimism that we will have a choice of good vaccines in the next few months.
“First we heard 90% efficacy from Pfizer and BioNTech, then the Russians said 92% and now Moderna says 94.5%.
“This latest press release is based on a study of 30,000 US adults, including many high-risk or elderly persons.
“This gives us confidence that the results are relevant in the people who are most at risk of Covid-19 and in most need of the vaccines.
“Moderna have also announced that the vaccine can be kept in a conventional freezer (-20C) for up to six months, and that once thawed the vaccine can be kept for up to 30 days at standard refrigeration (2 to 8C). This makes the vaccine much easier to deliver.”
The Pfizer/BioNTech vaccine needs to be kept at minus 70C (minus 94F) before being transferred to a fridge, which could pose transport and storage issues.
Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene and Tropical Medicine, said: “As with previous announcements, this is encouraging for other vaccines that may be expected to also have worthwhile efficacy.
“Although they reported efficacy being over 94%, there is statistical uncertainty in this, but based on these data, the likely efficacy will be better than 85% which would be greater than most scientists would have expected.
“This is the first study to report on severe cases and while uncertainty remains, the finding of no severe cases with the vaccine and 11 cases with placebo is very strong evidence that the vaccine prevents severe as well as mild disease.”
He said more data was needed on the elderly “but this is definitely encouraging progress”.
President Ursula von der Leyen, head of the European Commission, said on Monday the EU had “concluded exploratory talks with Moderna” and “we hope to finalise the contract soon”.
Liberal Democrat health spokeswoman Munira Wilson tweeted the vaccine news was “fantastic” but added: “Shame we weren’t part of the EU vaccine procurement prog, or we’d have early access to the Moderna vaccine, as well as Pfizer vaccine. I do hope UK Govt can secure a good deal at this late stage. I daresay Moderna’s negotiating position just got a lot stronger!”




Comments: Our rules
We want our comments to be a lively and valuable part of our community - a place where readers can debate and engage with the most important local issues. The ability to comment on our stories is a privilege, not a right, however, and that privilege may be withdrawn if it is abused or misused.
Please report any comments that break our rules.
Read the rules hereLast Updated:
Report this comment Cancel